Plants and Their Essential Elements
All organisms must take in matter from their environment in order to survive. There are 92 naturally occurring elements on Earth. Living things need only a minority of them. For example, humans require about 21 different elements to be healthy. Almost all of the mass of our bodies comes from just six of those elements (carbon, hydrogen, oxygen, nitrogen, phosphorus , and calcium). These are the elements used to construct the carbohydrates, nucleic acids, proteins, and other molecules that make up our cells and carry out their chemistry. Other elements critical to our health are needed in very small amounts. Often, such elements are cofactors required by enzymes to catalyze specific chemical reactions. Regardless of whether elements are needed in large or small amounts, they must be obtained from the environment. Furthermore, it not enough that essential elements are present in the environment; they must be available in a chemical form that our bodies can use.
Not surprisingly, the situation in plants is similar. They, too, must carry out thousands of different chemical reactions, many of which are similar to those of humans. Scientists have identified 17 elements that are essential for plants (see the table below). An element is described as being essential to the plant if the following conditions are met:
- The element must be required by the plant to complete its life cycle.
- The element cannot be replaced by another element.
- The element must be required for a specific biological function.
- The element must be required by a substantial number of different plant species.
Essential elements can be classified as mineral or non-mineral nutrients. Carbon, hydrogen, and oxygen are classified as non-mineral nutrients because they are obtained from the atmosphere and water. Mineral nutrients can be further classified as being either macronutrients or micronutrients. As the name implies, macronutrients are needed in relatively large amounts. Nitrogen, phosphorous, and potassium are called primary macronutrients, while calcium, sulfur, and magnesium are called secondary macronutrients. The rest of the essential elements are called micronutrients because they are needed in small amounts. It is important to note that despite their name, micronutrients are just as essential to plant health as are macronutrients.
Plants absorb most of their essential elements from water in the soil. Usually the essential elements are taken up as a positively charged cation or a negatively charged anion.
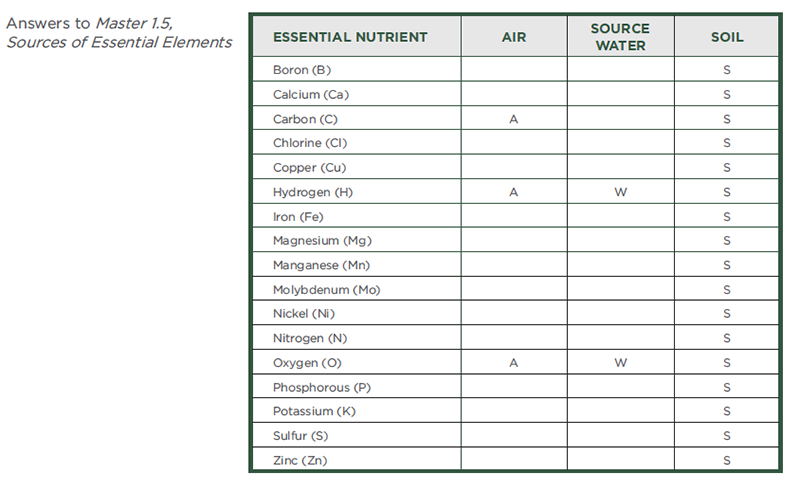
Essential Plant Nutrients
| Element |
Symbol |
Classification |
Chemical Form Taken into the Plant |
| Hydrogen |
H |
Nonmineral nutrient |
H2O |
| Oxygen |
O |
Nonmineral nutrient |
O2 and CO2 |
| Carbon |
C |
Nonmineral nutrient |
CO2 |
| Nitrogen |
N |
Primary macronutrient |
NH4+ and NO3- |
| Phosphorus |
P |
Primary macronutrient |
H2PO4- and H2PO42- |
| Potassium |
K |
Primary macronutrient |
K+ |
| Calcium |
Ca |
Secondary macronutrient |
Ca2+ |
| Magnesium |
Mg |
Secondary macronutrient |
Mg2+ |
| Sulfur |
S |
Secondary macronutrient |
SO42- |
| Boron |
B |
Micronutrient |
B(OH)3 |
| Chlorine |
Cl |
Micronutrient |
Cl- |
| Copper |
Cu |
Micronutrient |
Cu2+ |
| Iron |
Fe |
Micronutrient |
Fe2+ and Fe3+ |
| Manganese |
Mn |
Micronutrient |
Mn2+ |
| Molybdenum |
Mo |
Micronutrient |
MoO42- |
| Nickel |
Ni |
Micronutrient |
Ni2+ |
| Zinc |
Zn |
Micronutrient |
Zn2+ |
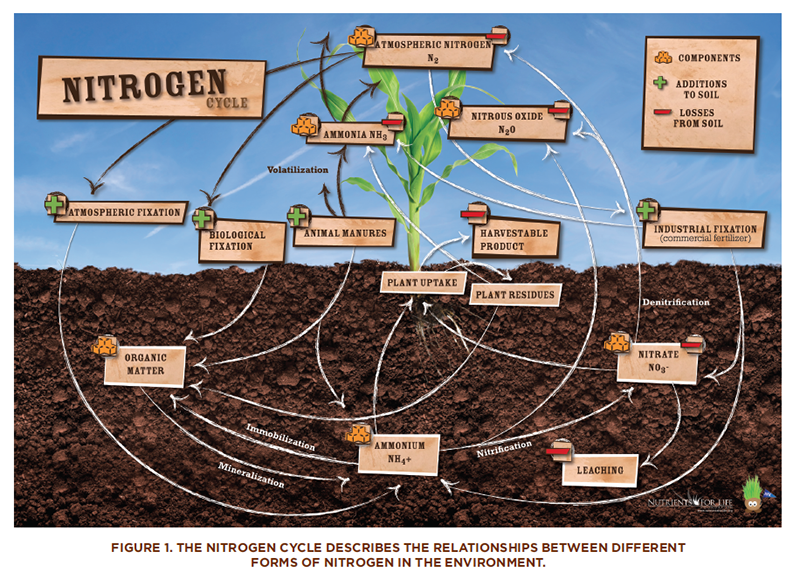
The Nitrogen Cycle
Although the atmosphere is about 78 percent nitrogen, plants cannot make use of nitrogen gas (N2). Instead, plants need to obtain their nitrogen by taking up the cation ammonium (NH4+) or the anion nitrate (NO3–) in the soil. These ionic forms of nitrogen are generated by the breakdown of organic material in the soil or through a process called nitrogen fixation that is carried out by soil microbes. Some crop plants (legumes such as peas, beans, peanuts, and soybeans) live in close association with nitrogen-fixing bacteria that live in their roots and convert N2 gas to a form that plants can use. Such crops have a steady source of nitrogen and do not require nitrogen-containing fertilizers.
The nitrogen cycle describes the processes by which nitrogen moves between its various chemical forms. Biological or physical processes can cause these chemical conversions. Four processes are essential to the nitrogen cycle.
- Nitrogen fixation refers to the process by which atmospheric nitrogen (N2) is converted to nitrogen–containing compounds that are usable by plants. Nitrogen fixation can be accomplished through the action of lightning or bacteria in the soil.
- Ammonification refers to the process by which bacteria and fungi convert decomposed nitrogen-containing compounds into ammonium ions (NH4+).
- Nitrification refers to the process by which bacteria convert ammonium ions into nitrite (NO2-). Other bacteria convert nitrite to nitrate (NO3-). This is important because nitrites can reach levels that are toxic to plants.
- Denitrification refers to the process by which bacteria convert nitrates back to N2.
So, let us summarize the nitrogen cycle. First, recall that plants cannot use the nitrogen in the air that is so plentiful. When plants and animals die and decompose, they add nitrogen to the soil. Bacteria in the soil convert the nitrogen into compounds that plants can use. Plants take in these nitrogen-containing compounds through their roots and use them to grow. Animals eat the plants, use the nitrogen, and return it to the soil when they die and decompose.