Types of Fats and Their Impact on Health
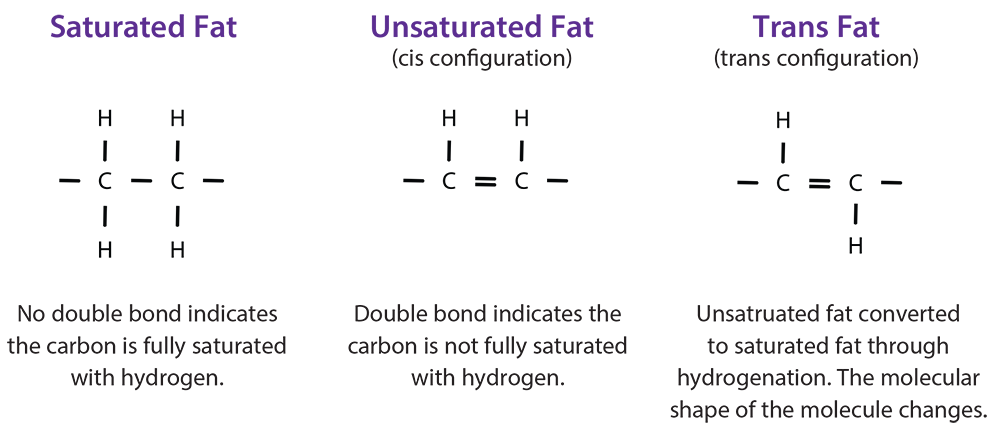
There are different types of fat including saturated, unsaturated, and trans (hydrogenated) fat. Unsaturated fats contain one or more double bonds. Saturated fats have no double bonds and are saturated with hydrogen. In excess, fats lead to many health problems like high blood pressure and stroke. Although trans fats can occur naturally in some products like butter, they are typically unsaturated fats that have been chemically altered (partially hydrogenated). When unsaturated fat is partially hydrogenated, a geometric isometric form of an unsaturated double bond is formed, resulting in trans fat. Margarine is an example of a partially hydrogenated fat. Because trans fats are typically not natural and are high in saturated fat, we should limit them in our diets. However, it is important to consume adequate amounts of fat in our diet because it is needed for energy, nerve and brain function, and growth. We should choose foods that are high in unsaturated fats, moderate in saturated fatty acids, and free of trans fats. Avoiding overconsumption of these foods will help decrease the risk for diet-related diseases such as heart disease, stroke, overweight/obesity, and Type 2 Diabetes.

Melting Points, Solidification, and Hydrogenation
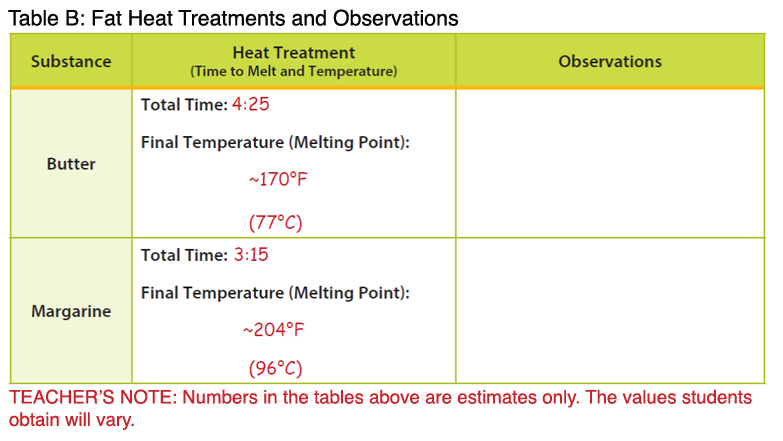
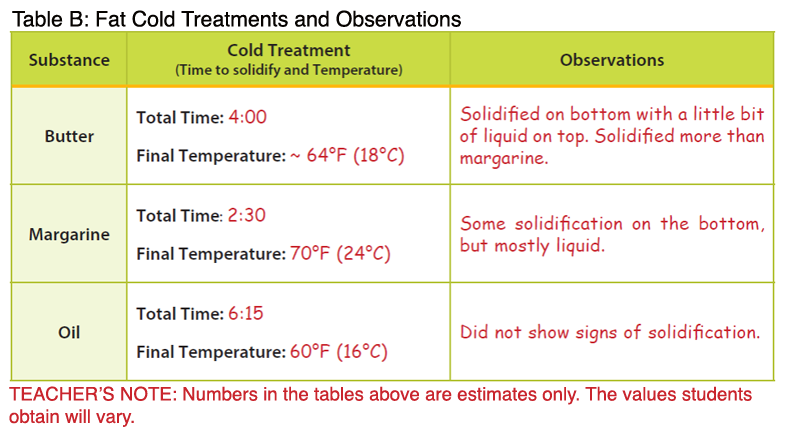
Many students misunderstand chemical and physical changes. Students may think a change of state is chemical, when it is truly physical. You can use the concepts of melting point and solidification to correct these misconceptions in a hands-on, visual manner. Melting point is the point at which a solid fat turns to liquid. It varies from food to food. Solidification, or freezing, is the point at which a liquid turns to a solid. Butter melts at 90-95°F; however, there is no set point for butter to solidify because of its complexity. The exact fat/fatty acid composition is not the same from product to product. Butter will usually solidify around 60-65°F. The melting point of margarine is slightly higher than butter because of the presence of hydrogenated fats. It melts at 94-98 °F. Like butter, there is no set point at which it will solidify; it will usually solidify around 65-70°F. Solid fats have different melting points because they are not pure substances, but rather combinations of mixed triglycerides. Triglycerides are considered the major form of fat and is the storage form of fat in our bodies. Triglycerides are made up of 3 fatty acid chains attached to a glycerol backbone.
Fats in the form of oil also have melting points; however, they are much lower than fats that are solid at room temperature. The melting points of oils vary based on their fat content. For example, the melting point of olive oil is 21°F, but peanut oil is much higher at 28°F. In general, the lower the melting point, the healthier the oil. Based on this information, olive oil would be the better choice. Oils can be converted to solids through a process called hydrogenation, in which all or some (partial hydrogenation) are converted to saturated bonds by the addition of hydrogen. Hydrogenation is a chemical process in which hydrogen atoms are added to the unsaturated carbons, making them saturated. The process occurs only at very high temperatures and results in the production of trans fatty acids (cis converted to trans configuration) among remaining unsaturated bonds in partially hydrogenated oils. Foods higher in hydrogenated fats, or trans fats, have higher melting points. Some factors that influence melting points include the number of carbons in the molecule, the degree of unsaturation, and the molecular configuration (cis versus trans). Fats with longer carbon chains have higher melting points.
Emulsions, Fat Polarity, and Cell Membranes
Heterogeneous mixtures have more than one color, substance, or texture visible. Homogeneous mixtures are the same throughout with a uniform appearance. An emulsion is a mixture of two immiscible liquids, like water and oil or vinegar and oil, where one is dispersed in small globules throughout the other. Immiscible liquids are not able to mix without an emulsifier that will create an emulsion. Emulsions are considered a heterogeneous mixture. Two liquids that are immiscible have opposite polarity. A liquid is either polar or non-polar. Polar liquids are miscible in other polar liquids and non-polar liquids are miscible in other non-polar liquids. In other words, miscible liquids will readily mix with each other without the help of an emulsifier. On the other hand, polar and non-polar liquids do not mix together without an emulsifier. When fats, typically oils, are mixed with an emulsifier, such as eggs, they form an emulsion.
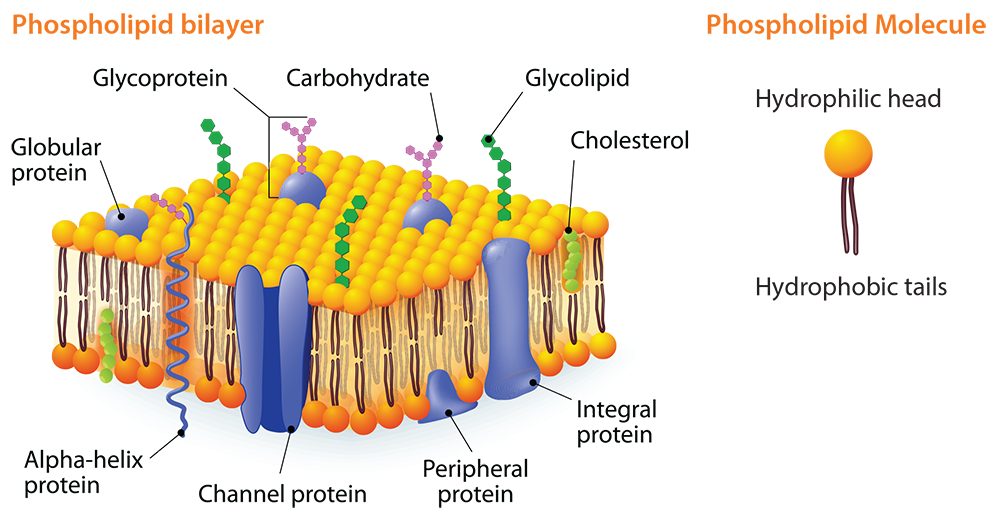
The concept of fat and oil polarity can be used to teach students about the cell membrane. A major component of a cell’s membrane is phospholipids. A phospholipid molecule has one end that is hydrophilic (water-loving). The other end is hydrophobic (water-fearing). The cell membrane has two phospholipid layers known as the phospholipid bi-layer. The hydrophilic heads of one layer face outward toward the extracellular environment and, in the other layer, they face inward toward the cytoplasm. This double layer is semi-permeable and only certain types of molecules can diffuse across the membrane.
FoodMASTER Middle Lessons
FoodMASTER (Food, Math and Science Teaching Enhancement Resource) is a compilation of programs aimed at using food as a tool to teach mathematics and science. For more information see the Background & Introduction to FoodMASTER for Middle School. This lesson is one in a series of lessons designed for middle school: